Two updates: 1) Treat endothelial dysfunction with sleep.
- heartlung
- Jan 16, 2023
- 3 min read
2) High blood sugar causes endothelial dysfunction via sympathetic over-activity
J Clin Atherosclerosis. 2017 Aug 18;265:41-46. doi: 10.1016/j.atherosclerosis.2017.08.001. [Epub ahead of print] Insufficient sleep is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation. Bain AR1, Weil BR2, Diehl KJ2, Greiner JJ2, Stauffer BL3, DeSouza CA4. Author information 1Integrative Vascular Biology Laboratory, Department of Integrative Physiology, University of Colorado, Boulder, CO 80309, USA. Electronic address: Anthony.bain@colorado.edu.2Integrative Vascular Biology Laboratory, Department of Integrative Physiology, University of Colorado, Boulder, CO 80309, USA.3Integrative Vascular Biology Laboratory, Department of Integrative Physiology, University of Colorado, Boulder, CO 80309, USA; Department of Medicine, University of Colorado Denver Anschutz Medical Center, Aurora, CO 80045, USA; Department of Cardiology, Denver Health Medical Center, Denver, CO 80204, USA.4Integrative Vascular Biology Laboratory, Department of Integrative Physiology, University of Colorado, Boulder, CO 80309, USA; Department of Medicine, University of Colorado Denver Anschutz Medical Center, Aurora, CO 80045, USA. Abstract BACKGROUND AND AIMS: Habitual short nightly sleep duration is associated with increased atherosclerotic cardiovascular disease risk and morbidity. Vascular endothelial dysfunction represents an important mechanism that may underlie this heightened cardiovascular risk. Impaired endothelium-dependent vasodilation, particularly NO-mediated vasodilation, contributes to the development and progression of atherosclerotic vascular disease and acute vascular events. We tested the hypothesis that chronic insufficient sleep is associated with impaired NO-mediated endothelium-dependent vasodilation in middle-aged adults. METHODS: Thirty adult men were studied: 15 with normal nightly sleep duration (age: 58 ± 2 y; sleep duration: 7.7 ± 0.2 h/night) and 15 with short nightly sleep duration (55 ± 2 y; 6.1 ± 0.2 h/night). Forearm blood flow (FBF) responses to intra-arterial infusion of acetylcholine, in the absence and presence of the endothelial NO synthase inhibitor NG-monomethyl-L-arginine (L-NMMA), as well as responses to sodium nitroprusside, were determined by strain-gauge venous occlusion plethysmography. RESULTS: The FBF response to acetylcholine was lower (∼20%; p<0.05) in the short sleep duration group (from 4.6 ± 0.3 to 11.7 ± 1.0 ml/100 ml tissue/min) compared with normal sleep duration group (from 4.4 ± 0.3 to 14.5 ± 0.5 ml/100 ml tissue/min). L-NMMA significantly reduced the FBF response to acetylcholine in the normal sleep duration group (∼40%), but not the short sleep duration group. There were no group differences in the vasodilator response to sodium nitroprusside. CONCLUSIONS: These data indicate that short nightly sleep duration is associated with endothelial-dependent vasodilator dysfunction due, in part, to diminished NO bioavailability. Impaired NO-mediated endothelium-dependent vasodilation may contribute to the increased cardiovascular risk with insufficient sleep. J Clin Hypertens (Greenwich). 2017 Sep 4. doi: 10.1111/jch.13060. [Epub ahead of print] The role of increased glucose on neurovascular dysfunction in patients with the metabolic syndrome. Rodrigues S1, Cepeda FX1, Toschi-Dias E1, Dutra-Marques ACB1, Carvalho JC1, Costa-Hong V1, N Alves MJN1, Rondon MUPB2, Bortolotto LA1, Trombetta IC3. Author information 1Heart Institute (InCor), Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil.2School of Physical Education and Sports, University of São Paulo, São Paulo, Brazil.3Universidade Nove de Julho (UNINOVE), São Paulo, Brazil. Abstract Metabolic syndrome (MetS) causes autonomic alteration and vascular dysfunction. The authors investigated whether impaired fasting glucose (IFG) is the main cause of vascular dysfunction via elevated sympathetic tone in nondiabetic patients with MetS. Pulse wave velocity, muscle sympathetic nerve activity (MSNA), and forearm vascular resistance was measured in patients with MetS divided according to fasting glucose levels: (1) MetS+IFG (blood glucose ?100 mg/dL) and (2) MetS-IFG (<100 mg/dL) compared with healthy controls. Patients with MetS+IFG had higher pulse wave velocity than patients with MetS-IFG and controls (median 8.0 [interquartile range, 7.2-8.6], 7.3 [interquartile range, 6.9-7.9], and 6.9 [interquartile range, 6.6-7.2] m/s, P=.001). Patients with MetS+IFG had higher MSNA than patients with MetS-IFG and controls, and patients with MetS-IFG had higher MSNA than controls (31±1, 26±1, and 19±1 bursts per minute; P<.001). Patients with MetS+IFG were similar to patients with MetS-IFG but had higher forearm vascular resistance than controls (P=.008). IFG was the only predictor variable of MSNA. MSNA was associated with pulse wave velocity (R=.39, P=.002) and forearm vascular resistance (R=.30, P=.034). In patients with MetS, increased plasma glucose levels leads to an adrenergic burden that can explain vascular dysfunction. ©2017 Wiley Periodicals, Inc. KEYWORDS: Diabetes mellitus; arterial compliance; endothelial function; metabolic syndrome; sympathetic nervous system Endothelial Function Scientific Update Sponsored by Endothelix Inc.
![Lipoprotein(a) levels predict endothelial dysfunction in maintenance hemodialysis patients: evidence from [VENDYS] vascular reactivity index assessment](https://static.wixstatic.com/media/dac531_5285607cc591409a9d83746f042af7c6~mv2.png/v1/fill/w_980,h_980,al_c,q_90,usm_0.66_1.00_0.01,enc_avif,quality_auto/dac531_5285607cc591409a9d83746f042af7c6~mv2.png)
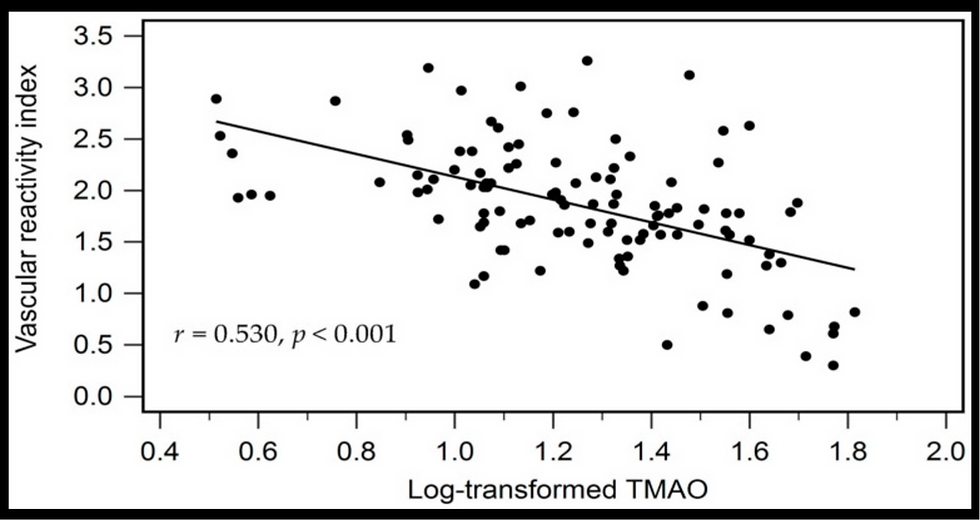

Comments