Body Shape and Endothelial Dysfunction -Data from a large Japanese study
- heartlung
- Jan 16, 2023
- 2 min read
Updated: Jan 18, 2023
Sci Rep 2021 Sep 9;11(1):17873 A body shape index is associated with endothelial dysfunction in both men and women Masato Kajikawa 1, Tatsuya Maruhashi 2, Shinji Kishimoto 2, Takayuki Yamaji 3, Takahiro Harada 3, Yu Hashimoto 3, Yiming Han 2, Aya Mizobuchi 2, Gaku Aoki 4, Kenichi Yoshimura 4, Kazuaki Chayama 5, Chikara Goto 6, Farina Mohamad Yusoff 2, Ayumu Nakashima 7, Yukihito Higashi 8 9 Abstract A body shape index (ABSI) was proposed for estimation of abdominal adiposity. ABSI has been reported to have associations with cardiovascular risk factors and cardiovascular events. However, there is no information on the association between ABSI and endothelial function. We examined cross-sectional associations of ABSI with endothelial function in 8823 subjects (6773 men and 2050 women). Subjects with a lower quartile of flow-mediated vasodilation (FMD) were defined as subjects having endothelial dysfunction. Pearson’s correlation coefficient analysis revealed that ABSI was negatively correlated with FMD (men, r = – 0.23, P = 0.003; women, r = – 0.32, P < 0.001). The areas under the curves of ABSI and body mass index to predict endothelial dysfunction were 0.64 (95% confidence interval [CI] 0.62-0.65) and 0.58 (95% CI 0.57-0.60) in men, and 0.68 (95% CI 0.66-0.71) and 0.59 (95% CI 0.56-0.61) in women, respectively. The cutoff values of ABSI for predicting subjects with endothelial dysfunction were 0.0796 (sensitivity, 55.2%; specificity, 65.5%) in men and 0.0823 (sensitivity, 56.2%; specificity, 73.4%) in women. Multivariate analysis revealed that an ABSI value higher than the cutoff value remained an independent predictor of endothelial dysfunction in both sexes. The results of our study suggest that ABSI calculation should be performed for evaluation of risk of cardiovascular events in both men and women. Clinical trial registration information URL for Clinical Trial: https://www.umin.ac.jp/ctr/index.htm ; Registration Number for Clinical Trial: UMIN000012952 (01/05/2010).

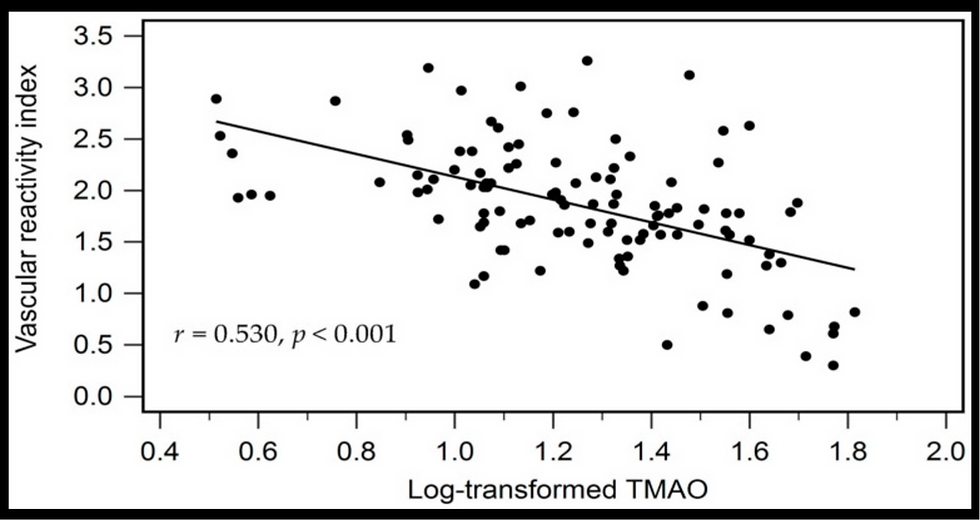
![Lipoprotein(a) levels predict endothelial dysfunction in maintenance hemodialysis patients: evidence from [VENDYS] vascular reactivity index assessment](https://static.wixstatic.com/media/dac531_5285607cc591409a9d83746f042af7c6~mv2.png/v1/fill/w_980,h_980,al_c,q_90,usm_0.66_1.00_0.01,enc_avif,quality_auto/dac531_5285607cc591409a9d83746f042af7c6~mv2.png)

Comments