Getting Old through the Blood:
- heartlung
- Jan 16, 2023
- 4 min read
Circulating Molecules in Aging and Senescence of Cardiovascular Regenerative Cells
Front Cardiovasc Med. 2017 Oct 6;4:62. doi: 10.3389/fcvm.2017.00062. eCollection 2017. Getting Old through the Blood: Circulating Molecules in Aging and Senescence of Cardiovascular Regenerative Cells. Angelini F1, Pagano F1, Bordin A1, Picchio V1, De Falco E1, Chimenti I1. Author information 1 Department of Medical Surgical Sciences and Biotechnologies, “La Sapienza” University of Rome, Latina, Italy. Abstract Global aging is a hallmark of our century. The natural multifactorial process resulting in aging involves structural and functional changes, affecting molecules, cells, and tissues. As the western population is getting older, we are witnessing an increase in the burden of cardiovascular events, some of which are known to be directly linked to cellular senescence and dysfunction. In this review, we will focus on the description of a few circulating molecules, which have been correlated to life span, aging, and cardiovascular homeostasis. We will review the current literature concerning the circulating levels and related signaling pathways of selected proteins (insulin-like growth factor 1, growth and differentiation factor-11, and PAI-1) and microRNAs of interest (miR-34a, miR-146a, miR-21), whose bloodstream levels have been associated to aging in different organisms. In particular, we will also discuss their potential role in the biology and senescence of cardiovascular regenerative cell types, such as endothelial progenitor cells, mesenchymal stromal cells, and cardiac progenitor cells. KEYWORDS: cardiac cell therapy; cardiac progenitor cells; cardiovascular regeneration; cell senescence; endothelial progenitor cells; insulin-like growth factor 1 fcvm-04-00062-g002.jpg
Physiol Rep. 2017 Nov;5(20). pii: e13478. doi: 10.14814/phy2.13478. Prolonged leg bending impairs endothelial function in the popliteal artery. Walsh LK1, Restaino RM2, Martinez-Lemus LA2,3, Padilla J4,3,5. Author information 1 Nutrition and Exercise Physiology, University of Missouri, Columbia, Missouri. 2 Medical Pharmacology and Physiology, University of Missouri, Columbia, Missouri. 3 Dalton Cardiovascular Research Center, University of Missouri, Columbia, Missouri. 4 Nutrition and Exercise Physiology, University of Missouri, Columbia, Missouri padillaja@missouri.edu. 5 Child Health University of Missouri, Columbia, Missouri. Abstract Uninterrupted sitting blunts vascular endothelial function in the lower extremities; however, the factors contributing to this impairment remain largely unknown. Herein, we tested the hypothesis that prolonged flexion of the hip and knee joints, as it occurs during sitting, and associated low shear stress and disturbed (i.e., turbulent) blood flow caused by arterial bending, impairs endothelial function at the popliteal artery. Bilateral measurements of popliteal artery flow-mediated dilation (FMD) were performed in 12 healthy subjects before and after a 3-h lying-down period during which one leg was bent (i.e., 90-degree angles at the hip and knee) and the contralateral leg remained straight, serving as internal control. During the 3-h lying down period, the bent leg displayed a profound and sustained reduction in popliteal artery blood flow and mean shear rate; whereas a slight but steady decline that only became significant at 3 h was noted in the straight leg. Notably, 3 h of lying down markedly impaired popliteal artery FMD in the bent leg (pre: 6.3 ± 1.2% vs. post: 2.8 ± 0.91%; P < 0.01) but not in the straight leg (pre: 5.6 ± 1.1% vs. post: 7.1 ± 1.2%; P = 0.24). Collectively, this study provides evidence that prolonged bending of the leg causes endothelial dysfunction in the popliteal artery. This effect is likely secondary to vascular exposure to low and disturbed blood flow resulting from arterial angulation. We conclude that spending excessive time with legs bent and immobile, irrespective of whether this is in the setting of sitting or lying-down, may be disadvantageous for leg vascular health. © 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society. KEYWORDS: Blood flow; endothelial dysfunction; shear stress; sitting vasculopathy J Ren Nutr. 2017 Nov;27(6):465-469. doi: 10.1053/j.jrn.2017.04.012. Skin Blood Flow and Vascular Endothelium Function in Uremia. Smogorzewski MJ1. Author information 1 Division of Nephrology and Hypertension, Department of Medicine at Keck School of Medicine, University of Southern California, Los Angeles, California. Electronic address: smogorze@usc.edu. Abstract Prevalence of dermatological disorder in patients with end-stage kidney disease is estimated as 50% to 100% in various studies. Some of the skin lesions are specific for the diseases causing chronic kidney disease (CKD), some are associated with CKD, and still others are the dermatological manifestation of uremia. Microangiopathy was also found in both arterioles and venule in the skin biopsy of "normal looking" skin in patients with end-stage kidney disease. In a cross-sectional study in patients on dialysis, we measured skin blood flow (SBF) using laser Doppler device in a standardized way at various areas of lower extremities at 2 different local skin temperatures: 35°C and 44°C. Local heating increases skin perfusion by mechanisms dependent on nitric oxide (NO). SBF was impaired in CKD patients Stage 5 on HD, particularly in those with diabetes mellitus as a cause of CKD. The reduced response in the SBF to the heat in our patients may be due to decreased generation of NO in uremia. Endothelium-dependent vasodilatation in patients on dialysis and the response of the skin microcirculation to acetylcholine was diminished in hypertensive patients on dialysis. Similarly, patients with diabetes mellitus had decreased SBF during intradermal microdialysis with a NO synthase inhibitor. Multiple uremic toxins have been studied in vitro and show to cause various degree of endothelial cell dysfunction. Unfortunately, no clear benefit has been described in CKD patients to different intervention aimed to reduce uremic toxin effect on endothelium. There are no long-term data on the factors which can modify endothelium function in uremia, but non pharmacologic interventions, diet, and several pharmacologic approaches could be beneficial. Measurement of SBF can be useful in evaluation of vasculopathy in CKD population and can potentially be used for assessment of vascular response during specific clinical intervention. Copyright © 2017. Published by Elsevier Inc. Endothelial Function Scientific Update Sponsored by Endothelix Inc.
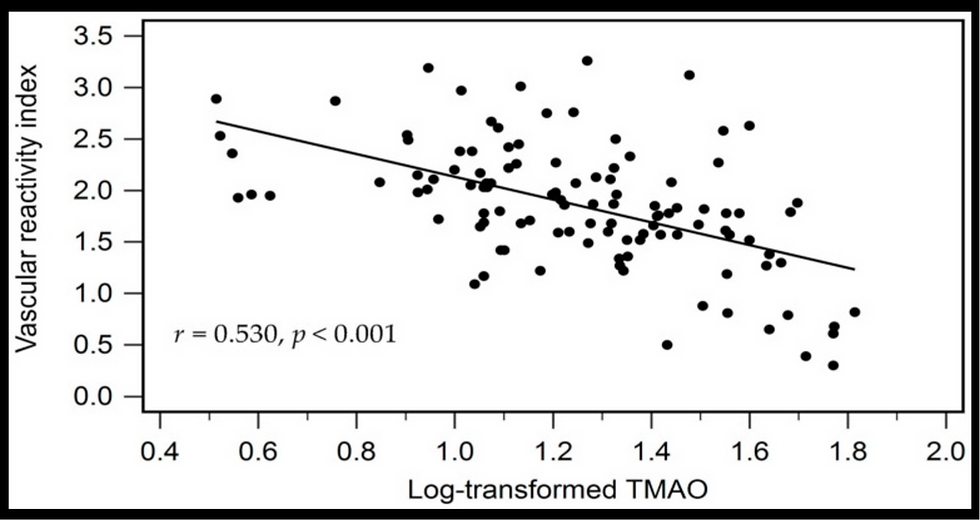
![Lipoprotein(a) levels predict endothelial dysfunction in maintenance hemodialysis patients: evidence from [VENDYS] vascular reactivity index assessment](https://static.wixstatic.com/media/dac531_5285607cc591409a9d83746f042af7c6~mv2.png/v1/fill/w_980,h_980,al_c,q_90,usm_0.66_1.00_0.01,enc_avif,quality_auto/dac531_5285607cc591409a9d83746f042af7c6~mv2.png)

Comments